LOPBURI, Thailand — When Gustun Aunlamai arrived at school at age 4, he was so overweight that his teacher worried he’d have trouble breathing during naptime. His arms and legs were thick. His mouth peeked out from two ballooning cheeks. He moved slowly.
Throughout his toddler years, Gustun had regularly asked his parents to refill his bottle with his favorite “milk” — a type of formula made especially for kids his age. And they were happy to oblige. Sumet Aunlamai and Jintana Suksiri, who lived in a rural province north of Bangkok, had carefully chosen the brand.
Like other Thai parents, they’d been bombarded by formula advertising on television, online and in grocery stores, where a rainbow of boxes and canisters of powdered toddler milk featured teddy bears in graduation caps and giveaways like toys or diapers. It cost far more than cow’s milk but promised to make Gustun stronger and smarter.
What Jintana didn’t know, as Gustun chugged the formula and his weight neared 70 pounds, was that her son’s choice drink had sparked an international feud.


In 2017, Thai health experts tried to stop aggressive advertising for all formula — including that made for toddlers. Officials feared company promotions could mislead parents and even persuade mothers to forgo breastfeeding, depriving their children of the vital health benefits that come with it. At the time, Thailand’s breastfeeding rate was already among the lowest in the world.
But the $47 billion formula industry fought back, enlisting the help of a rich and powerful ally: the United States government.
Over 15 months, U.S. trade officials worked closely with formula makers to wage a diplomatic and political pressure campaign to weaken Thailand’s proposed ban on formula marketing, a ProPublica investigation found.
U.S. officials delivered a letter to Bangkok asking pointed questions, including whether the legislation was “more trade restrictive than necessary.” They also lodged criticisms in a bilateral trade meeting with Thai authorities and on the floor of the World Trade Organization, where such complaints can lead to costly legal battles.
Thai officials argued the new regulation would protect mothers and babies. In the end, though, the Thai government backed down. It banned advertising for infant formula but allowed companies to market formula for toddlers like Gustun — one of the industry’s most profitable and dubious products. The final law also slashed penalties for violators.
“Our law is really weak and enforcement is really weak,” said Dr. Siriwat Tiptaradol, who championed the proposed ban as a former adviser to Thailand’s health minister, in an interview in Bangkok. “I was upset and disappointed.”

The U.S. endeavor in Thailand was part of a decadeslong, global effort to protect the United States’ significant formula production and export business. ProPublica reviewed thousands of pages of emails and memos by U.S. officials, letters to foreign ministries, correspondence from industry groups and academic research. We also interviewed health experts and government leaders in nearly two dozen countries, including former U.S. officials.
Together, the reporting shows the U.S. government repeatedly used its muscle to advance the interests of multinational baby formula companies, such as Mead Johnson and Abbott, while thwarting the efforts of Thailand and other developing countries to safeguard the health of their youngest children.
Just last March, at a meeting in Dusseldorf, Germany, U.S. officials opposed a reference to formula advertising bans in a new international food standard for toddler milk. The move came after industry lobbying.
At the center of many efforts was the Office of the United States Trade Representative, which advises the president on trade policy. Emails show its staff in regular contact with formula makers and their industry groups through meetings, calls and position papers — which the industry used to hammer its objections to regulations around the world. “Mead Johnson and other infant formula producers have been very vocal, expressing concerns to the Thai and U.S. governments about what they feel is the imminent passage of this measure,” U.S. officials wrote in 2016 as Thailand considered its formula marketing ban.
Officials with the USTR and other trade-focused agencies, including those within the U.S. Department of Agriculture, then echoed those positions in communications with other countries or in international forums like the WTO, the documents showed.
“The U.S. is highly influential,” said Dr. Robert Boyle, a doctor at Imperial College London who has researched international formula use.
In many places, the lobbying appeared to succeed. Hong Kong, for example, watered down some of its formula regulations after objections from U.S. trade officials, who said in a draft letter that the rules “could result in significant commercial loss for U.S. companies.” And a proposal in Indonesia stalled after questions from the U.S. at the WTO.
Notably, such advocacy has not only hindered local attempts to stop formula marketing that critics say is misleading or even predatory, but it has also undermined the work of U.S. foreign aid and health officials, who have long supported breastfeeding across the globe. They call it “one of the highest returns on investment of any development activity” because of its well-documented benefits for babies’ health and cognitive growth.
“I think it is shocking,” said Jane Badham, an independent nutrition consultant and expert in child feeding who works internationally. “One doesn’t realize how much this kind of interference is happening.”
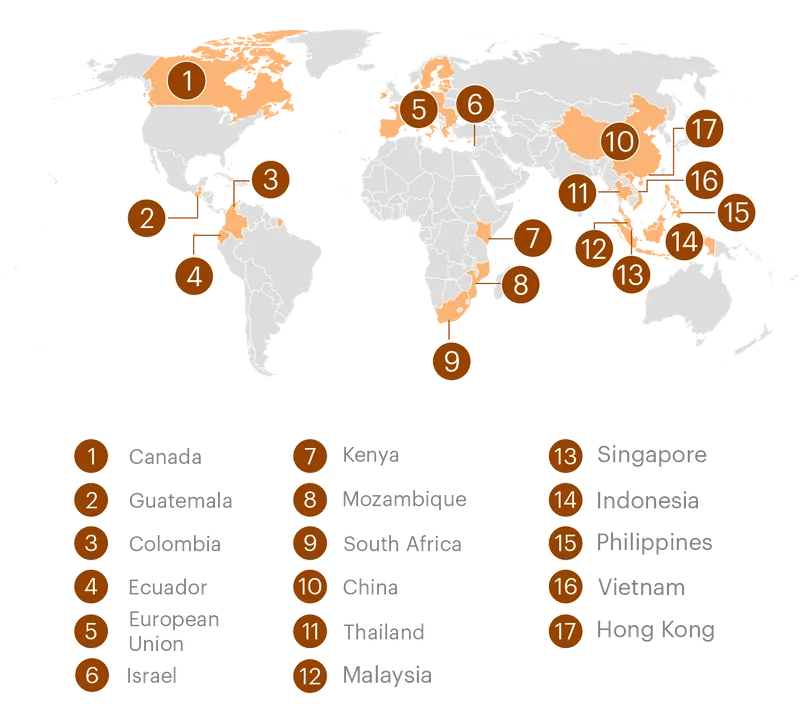
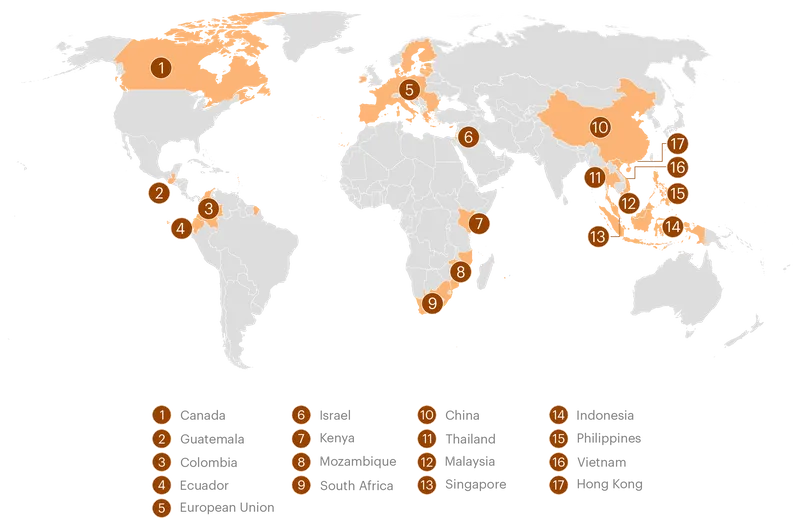
The meddling broke into public view in 2018, when officials from the Trump administration were accused of threatening to withhold military aid from Ecuador if the country didn’t drop its proposed resolution in support of breastfeeding at the World Health Organization; the U.S. ambassador later denied making threats. But ProPublica’s investigation found that the scope of the interference far exceeded that incident and continues today under the Biden administration. In fact, Ecuador and Thailand were just two stops on a worldwide crusade against regulation that has spanned Republican and Democratic presidential administrations and touched more than a dozen countries, including South Africa, Guatemala and Kenya, as well as Southeast Asian nations such as the Philippines, Malaysia and Vietnam.
The U.S. Has Waged a Global Campaign Against Formula Regulation
U.S. agencies have intervened in at least 17 jurisdictions over the last several decades on behalf of the formula industry, often to oppose measures that would restrict formula marketing or require additional safety precautions.
Neither Abbott nor Mead Johnson responded to requests for interviews or to detailed questions from ProPublica. The latter’s parent company, Reckitt, also did not respond to our request for comment.
USTR officials declined to be interviewed for this story. In response to written questions, an agency spokesperson said in a statement that under President Joe Biden, the trade agency has emphasized respecting the role of foreign governments in deciding the appropriate regulatory approach, including with respect to infant formula. USTR has been committed “to making sure our trade policy works for people — not blindly advancing the will of corporations,” the statement said.
That has meant moving the office “away from the formerly standard view that too often deemed legitimate regulatory initiatives as trade barriers,” the spokesperson said, adding that the move has “enervated” corporate players who have been used to “getting their way at USTR for decades.”
The spokesperson, however, declined to provide examples of the new approach in relation to formula. She also declined to respond to questions about government documents that show the trade office under Biden working with other federal agencies to pursue the same playbook on formula as prior administrations.
In 2021, for example, officials complained to Filipino trade authorities about stricter formula marketing rules they considered “overkill,” and expressed fears about regulatory “spillover” elsewhere in Southeast Asia. In Kenya, they sought to strike a provision in a proposed formula advertising ban after an industry group sent USTR a paper seeking its deletion.
Public health officials are increasingly raising concerns about toddler milk, especially as companies deploy advertising for products using bold — and, critics say, often unsupported — health claims.
In October, the American Academy of Pediatrics published a new report warning about the marketing for toddler formula. “Products that are advertised as ‘follow-up formulas,’ ‘weaning formulas’ or ‘toddler milks and formulas’ are misleadingly promoted as a necessary part of a healthy child’s diet,” said Dr. George Fuchs III, a lead author of the study. The drinks are worse than infant formula for babies under 1 year, he said, and “offer no benefit over much less expensive cow’s milk in most children older than age 12 months.”


Unlike infant formula, toddler milks are not regulated by the Food and Drug Administration. Nutrition experts have warned about hefty doses of sweeteners and sodium in some brands.
The Infant Nutrition Council of America, a formula industry group, defended toddler drinks, saying they “can contribute to nutritional intake and potentially fill nutrition gaps for children 12 months and older.”
Toddler milk made up just 11% of all formula sales in the United States in 2023, but it was much more popular abroad, according to Euromonitor, which tracks sales data. Worldwide, it made up 37% of sales. In Thailand, it accounted for more than half.
The country is now struggling to address the consequences of the law’s weakening, researchers and officials say. More than 1 in 10 Thai children under 5 years old face what researchers call a “double burden of malnutrition” that leaves some struggling with obesity and others lagging behind growth targets. Increased breastfeeding could help address both problems.
“You go to school and see a lot of kids are overweight,” said Dr. Somsak Lolekha, president of the Royal College of Pediatricians of Thailand and the Pediatric Society of Thailand. “We have a big problem in Thailand.”
Targeting “the Sippy Cups of the World”
Formula is one of only two products with international recommendations to prohibit its marketing. The other is tobacco.
The warning dates to 1981, when the nations that make up the governing body of the WHO passed the International Code of Marketing of Breast-Milk Substitutes. It aimed to stop all promotion of drinks meant to replace breast milk.
The move followed reports in the 1970s that thousands of infants in impoverished countries were falling ill and dying after drinking formula.



Not only were mothers using costly formula to replace breast milk, which would have given their babies better immunity, but the water parents mixed milk powder with was sometimes contaminated, leading to life-threatening bacterial infections and diarrhea. Overdiluted formula was causing severe malnutrition, too. Activists called for a boycott of the world’s biggest formula maker, Nestlé, which had heavily promoted its products in developing countries.
During the height of the controversy, an average 212,000 babies in low- and middle-income countries died preventable deaths linked to formula use annually, an academic paper circulated by the National Bureau of Economic Research estimated last year. (Nestlé disputed the research and said it was the first formula company to incorporate the WHO recommendations into its marketing policy in 1982.)
The United States cast the sole “no” vote against the international code, with the Reagan administration citing First Amendment protections on advertising. The Washington Post quoted a senior federal official who resigned over the decision, saying it would be “seen in the world as a victory for corporate interests.”
To be sure, formula was crucial for babies who didn’t have access to breast milk. But for those who did, public health experts feared aggressive advertising and free samples would derail a critical cycle. Once babies start drinking formula regularly, research shows, their mothers’ breast milk supply can drop.
“The evidence is strong,” a WHO and UNICEF report explains. “Formula milk marketing, not the product itself, disrupts informed decision-making and undermines breastfeeding and child health.”
In the years since the international code was adopted, at least 144 countries have sought to enshrine its voluntary restrictions into laws that bar formula marketing in stores, hospitals and elsewhere. Despite poor enforcement in many places, the laws have had measurable benefits. Studies have shown that countries that adopted marketing bans saw their breastfeeding rates rise, and more breastfeeding is in turn linked to fewer infant deaths. It also reduces mothers’ risk of certain cancers.
Baby formula manufacturers responded to slower growth in infant formula sales by creating products for older babies and toddlers — age groups that fell outside most regulations.

“We have a proven global demand-creation model,” Greg Shewchuk, Mead Johnson’s head of global marketing, told investors in 2013. “Capture baby very early on, often before it’s born, hold onto them through feeding and their feeding challenges and extend them as long as possible.”
Mead, which was based in the United States until a British company bought it in 2017, termed the strategy A-R-E: Acquisition, Retention, Extension.


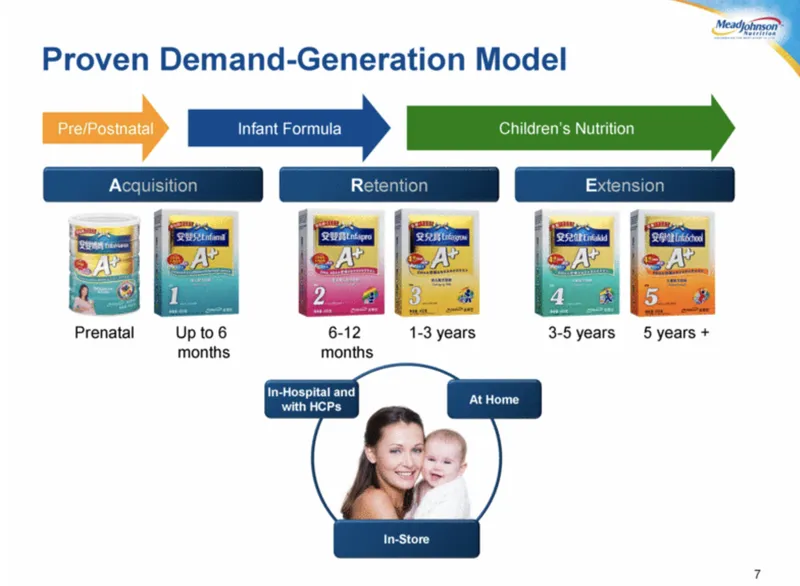
To make toddler products more attractive to parents, who usually just gave their kids cheaper cow’s milk beginning at age 1, formula makers began adding nutritional supplements like DHA, an omega-3 fatty acid found in fish and algae with purported benefits for brain and eye health.
The claims, however, are unproven. Studies have found no definitive link between babies’ brain and eye development and DHA supplementation, a 2017 meta-analysis of 15 studies found, according to Cochrane, a nonprofit that supports systematic reviews of health research. In fact, breastfed babies perform better on intelligence tests.
Infant Formula Looks Nearly Identical to Toddler Milk on a Grocery Store’s Shelves in Bangkok
Thailand's Milk Code restricts the advertisement of infant formula, but marketing of toddler milk is generally allowed.

Still, formula companies used additives like DHA “as a hook to expand their market share,” said Peter Buzy, CFO and treasurer of Martek Biosciences Corp., which produced DHA, at an analysts’ meeting in 2004. “Really targeting, you know, the sippy cups of the world.”
A spokesperson for the Infant Nutrition Council of America defended the health and nutrition claims, saying they “are based on science and medical research and meet all legal, regulatory and nutritional science requirements.”
The marketing worked. Toddler milk has overtaken infant formula in worldwide sales, according to Euromonitor. Global toddler milk sales have grown by 25% since 2013, to almost $20 billion. A little less than two gallons of toddler milk can cost $30 or more, compared with around $3.94 a gallon for regular milk in the U.S.
For formula manufacturers, the popularity of the product had another benefit: It helped them circumvent local rules against marketing infant formula. By using similar logos, colors or fonts across product lines, legal advertisements for toddler milk effectively promoted baby formula too, even in places where it was subject to a marketing ban. Nutrition experts and advocates called the tactic “cross-promotion.”
During the past decade, sales of regular infant formula grew about 10% worldwide, to $15 billion.
A Focus on Developing Nations
In 2014, Jintana gave birth to the couple’s first child, whom they nicknamed “Captain” after a soccer player.
The family lived in military housing in Lopburi, a rural province two hours north of Bangkok whose capital city is world famous for its flourishing monkey population. With Sumet serving in the Army, Jintana took time off from her job in customer relations to care for the newborn.
She breastfed Captain until it was time to return to work three months later. The couple shopped for formula. Health claims formula makers listed on packages were “very important,” Sumet said through a translator. They settled on a product called Dumex that promised to strengthen Captain’s brain, immunity and eyes. It was made by the French giant, Danone, which boasts that the brand “has happily raised generations of Thais.”

Millions of women like Jintana had been entering the workforce in developing regions such as Southeast Asia. The big six transnational companies that make most of the world’s baby formula saw this as a boon.
For Mead Johnson, the maker of Enfamil, the benefits of developing economies were twofold. “Firstly, in most countries, breastfeeding is incompatible with women participating fully in the workforce,” CEO Kasper Jakobsen said in a 2013 earnings call. “And, secondly, as women participate in the workforce, that creates a rapid increase in the number of dual-income families that can afford more expensive, premium nutrition products.”
By then, Thailand was Mead’s fifth-biggest market worldwide. And Southeast Asia was well on its way to becoming more important to the formula industry than the U.S. and European markets combined.
As business boomed, advocates lambasted the industry for its practices. Mead employees, for example, allegedly bribed health care workers at government hospitals in China so they would recommend the company’s formula to new mothers — charges the company ultimately resolved with a $12 million settlement in 2015; the company did not admit or deny regulators’ findings in the agreement. Danone faced similar allegations from Chinese media related to the brand Captain and Gustun drank, Dumex. Danone said at the time that it accepted responsibility for the lapses and suspended the program involved, according to the BBC.
The industry maintained close relationships with the medical establishment in Thailand, too. One pediatrician and advocate for breastfeeding, Dr. Sutheera Uerpairojkit, told ProPublica that two decades ago, she saw formula companies offer doctors and medical staff trips abroad in exchange for giving patients free samples and collecting their data. Some took the trips. Sutheera did not participate.

Thailand adopted the international code in 1984 — but only as a voluntary measure. Over the years, Siriwat and others pushed for tougher formula marketing restrictions without success. In one meeting, he and colleagues at the Thai health ministry pressed formula companies to comply with the voluntary rules, which they’d routinely broken. The businesses resisted. “One company said, ‘If I do not violate, I cannot compete with other companies,’” Siriwat recalled in September.
“That makes me very angry,” he said, remembering how he stormed out of the room.
By 2014, with Thailand’s breastfeeding rate at only 12%, according to one survey, Siriwat persuaded the health minister to seek legislation to formally ban marketing infant and toddler formula. He wanted the new law to include enforcement and penalties for violators.
The WHO, a United Nations agency promoting health, wanted more countries to pursue such measures. Its staff in 2016 released new recommendations on ending the promotion of formula products for toddlers, as well as infants. In theory, that guidance could help countries like Thailand fend off trade complaints about new marketing bans. And an endorsement by the WHO’s member nations would underscore the recommendations’ importance.
But public health wasn’t the only concern as nations prepared to vote.
U.S. Intervention on a Global Stage
The WHO effort alarmed formula makers, which worried that it would kick off a new round of laws against formula marketing. “That’s what’s at stake by a new measure that’s being proposed by the WHO, without any scientific evidence,” Audrae Erickson, a Mead Johnson lobbyist, told a trade association crowd.
Industry groups scrambled to arrange meetings with high-level officials in Washington. “Clearly, the potential economic and international trade implications from this proposed draft guidance are quite significant,” the pro-industry Infant Nutrition Council of America said in a letter to an FDA official in 2016.
That year, companies and trade groups connected to commercial milk formula, including Abbott Laboratories and Nestlé, spent almost $7 million lobbying U.S. officials about WHO matters, after a decade in which their lobbying disclosures had not mentioned the organization at all, a study found.
The industry’s outreach spanned Washington. The Infant Nutrition Council of America, for instance, lobbied the Senate, House and USTR — as well as the commerce, state, agriculture and health departments, lobbying records show. The efforts attracted the attention of leaders in both parties, including House Speaker Paul Ryan, who called President Barack Obama about the issue, according to records obtained by ProPublica.
Inside the administration, USTR took up the formula industry’s cause. “USTR does not support issuance of the guidance or resolution” on toddler milk, wrote Jennifer Stradtman, a USTR official, in an email to other federal officials. Furthermore, she wrote, her office “will not be able to accept” any resolution that encouraged WHO member countries to convert any of the guidance into law.
It wasn’t the first time the USTR sided with industry despite public health concerns: In 2013, a group of Democratic senators scolded U.S. Trade Representative Michael Froman for a proposal to help tobacco companies use trade law to “subvert” tobacco control measures — a stance the lawmakers called “deplorable and a serious threat to global public health.”
In the debate over toddler milk, officials from Froman’s office repeatedly questioned science, prompting a fight with public health officials, internal documents show.
In one exchange, then-USTR lawyer Sally Laing objected to a sentence from the guidance that said research suggests food preferences are established early in life.
“Unsupported,” Laing wrote.
Health officials pushed back on that, as well as other USTR edits. “MUST NOT DELETE,” the Centers for Disease Control and Prevention protested in all caps, arguing that key language in the resolution was, in fact, backed by scientific evidence. But such concerns appeared to get lost in the debate, as those sentences were ultimately struck from the text.
Meanwhile, as WHO member nations gathered to vote in Geneva, formula lobbyists had U.S. officials “on speed dial” and urged them to weaken the WHO resolution, said Jimmy Kolker, who led the negotiations for the U.S. as an assistant secretary in the Health and Human Services Department.
And the industry’s agents appeared to have inside knowledge. A baby-formula industry association lobbyist cornered Kolker. “From her approach, it was obvious to me that she had been forwarded an internal, very-limited-distribution USG email,” he wrote in an email to other U.S. government officials. “This is unacceptable and makes our job as negotiators significantly more difficult.”
In the end, the United States delegation persuaded WHO nations not to “endorse” their staffs’ own recommendations. Instead, the body voted only that it “welcomes with appreciation” the guidance — language that undercut its utility. The resolution, lacking the weight of an official endorsement, left many nations puzzled over whether it would help neutralize trade complaints.
“That has caused a lot of confusion,” said Laurence Grummer-Strawn, a WHO official who focuses on child feeding and former nutrition chief for the CDC. “What does that really mean?”
Stradtman and Laing could not be reached for comment. Froman did not respond to requests for comment, and a USTR spokesperson declined to comment on the office’s actions during the WHO debate. In a general statement, the spokesperson said that “with regard to infant formula, USTR, in conjunction with others in the interagency, work to uphold and advocate for policy and regulatory decisions that are based on science.”
The practical impact of the resolution’s weakened wording became clear within months, when the U.S. and other dairy producers like Australia and Canada accused Thailand of attempting to obstruct trade with its marketing ban. Thai officials argued their country had a “strong need for a regulation,” saying the “sales promotion” of milk formula for babies and toddlers contributed to the nation’s low rate of breastfeeding. But when it referenced the WHO’s guidance and resolution to support its position at the WTO, the U.S. countered that those measures did not amount to “an international standard.”
When the Thai National Legislative Assembly finally passed its formula marketing measure in April 2017, the provisions that the U.S. and its allies — plus some Thai doctors and industry lobbyists — had complained about most loudly were either watered down or gone entirely. Lawmakers had reduced the maximum criminal penalty for violations from three years in prison to one year in prison and the maximum fine from about $8,730 to $2,910, a USDA document shows.

The law banned the marketing of infant formula and outlawed cross-promotion, but it still allowed advertising on products for 1- to 3-year-olds.
At a June 2017 meeting of the WTO, the U.S. called the changes “a welcome modification.”
“Addicted to the Bottle”
The next year, Sumet and Jintana celebrated the birth of their second child, Gustun. As she had with her firstborn, Jintana breastfed Gustun until he was 3 months old, then started him on formula so she could go back to work.
The couple diligently followed the “stages” prescribed by Dumex, which came in a cheery red package: Stage 1 formula when Gustun was an infant, Stage 2 when he was an older baby and Stage 3 when he became a toddler. He craved formula, and his parents, believing it was healthy, always gave him more. By the time he was 3, he reached his peak weight of about 66 pounds — the same as an average 9-year-old. He was drinking six or seven bottles a day, each holding about 12 ounces of toddler milk.


Jintana wasn’t worried at first as Gustun grew pudgy. His brother, Captain, had been big, too — almost 60 pounds — at the same age. But when Gustun started school in person after the pandemic, his teachers were concerned. They had seen others arrive, as one put it, “addicted to the bottle.” The weight slowed Gustun down during movement time, his teacher Tida Rakrukrob said through a translator. “He would move slowly and was less active compared to other children,” she said.
When another teacher posted a video on TikTok showing herself comforting and talking with Gustun one day, it went viral — receiving 732,000 likes and many comments about how cute he was. But his teacher’s concern with his difficulty moving led his parents to bring him to see a doctor, who tested him for a hormone imbalance and checked him for diabetes. The tests came back negative. The parents reduced the fried food, dessert and snacks Gustun ate.
The biggest change the family made, though, was eliminating toddler formula from his diet. His school gave him cow’s milk instead, as it did for other children.
Gustun’s extra weight began to disappear.
Looking back, Jintana said she thinks he gained so much “because of the toddler milk.”
Today at age 6, Gustun is no longer on a restricted diet — he can eat fried food and dessert — and weighs 35 pounds, about half of what he weighed at the peak of his Dumex consumption. He is more outgoing at school, Jintana said, and plays soccer with his older brother every day. Captain lost a similar amount of weight after switching to cow’s milk at school and is now 9 and slim, weighing around 51 pounds.

One Monday in September, the brothers — both in soccer jerseys — kicked a ball back and forth in the driveway of the family’s brightly painted red house. Gustun, who has a lightning bolt shaved into his hairline, chased the ball and tried to get it away from his brother, who darted about quickly, tapping it from foot to foot.
“Now, his movement is perfect,” his mother said.
Danone, the company that makes Dumex, said in a statement that while breast milk offers children the best nutritional start, “50 years of scientific research into nutritional needs in early life underpins our products, and we do not make claims that have not been backed up by scientific research.” The company said that research has shown that toddler milk can provide nutrition and help improve the diet of children age 1 and older, reducing the risk of iron and vitamin D deficiency.
“We encourage parents to follow the guidelines on pack when using our products, which are carefully calibrated so that babies and infants receive the right amount of nutrients they need each day from our products,” the company said.
“The Tactic is ‘I Will Violate Your Law’”

Thailand’s marketing restrictions have done little to curb practices like cross-promotion, said Nisachol Cetthakrikul, who has worked in the Thai health ministry and studied the law.
Indeed, at two supermarkets in Bangkok, shiny walls of powdered formula boxes seven shelves high greeted shoppers on a warm day in September. There were few differences between packages for products intended for babies and those intended for toddlers.
Formula makers and stores offered steep discounts for toddler milk, calling one a “Mommy Fair Shock Deal.” An offer on one shelf told parents if they spent about $87 on Hi-Q1 toddler formula, made by Danone, they could receive a free yellow and blue swing set worth about $27. Other offers included a clay “pizza dough cooking fun set,” a toy keyboard and microphone, and even a pushable “speedcar trolley” that a toddler could sit in.
A 2022 study led by Nisachol found 227 instances of formula marketing that violated the law.
The government has levied fines for violations, but Thailand’s health ministry doesn’t name offenders. “The tactic is ‘I will violate your law,’” Siriwat said, “‘and prepare the budget for the fine.’”
Thai health authorities have tried to fight back by raising parents’ awareness of the benefits of breastfeeding. The health ministry, for example, erected billboards saying “breast milk is medicine” and called doctors to a meeting to urge them to promote breastfeeding among their patients. But these campaigns are no match for the formula companies’ massive spending on marketing, Siriwat said.

While Thailand’s exclusive breastfeeding rate for babies six months or younger rebounded to about 29% in 2022, UNICEF found, it is still far short of the WHO’s target of at least 50% by 2025. The country’s rates of obesity and stunting for children 5 and under are higher today than they were in 2016, the year before the watered-down formula law passed.
Dr. Somsak Lolekha, president of the Thai pediatric society, said formula isn’t the only reason for children’s weight problems. But it plays a big role, he said, because it’s so easy to drink — a point that tracks with studies showing that babies who breastfeed longer are less likely to become obese and develop diabetes than those who drink formula.

Last summer, Thailand joined more than 100 nations at the WHO’s headquarters in Geneva to explore ways to fight unethical formula marketing. Attendees sat at long tables in a sleek, modern auditorium. Like other nations’ representatives, Dr. Titiporn Tuangratananon, an official with Thailand’s health ministry, declared her intentions on brightly colored paper posted at the front of the room: “Fully control” the marketing of formula to young children, and “Increase + expand enforcement.”
In an interview, Titiporn said health officials are trying to update the country’s marketing rules — including making some forms of toddler formula advertising, such as giveaways, discounts and free samples, illegal.
But that could ultimately prove difficult in a country that is now the seventh-largest market in the world for formula.
In fact, according to Titiporn, the government has already been deluged by public comments critical of its regulatory efforts. She suspected the pro-marketing remarks, some of which had been repeatedly copied and pasted, came from representatives of the formula industry.
“We know that it’s not real,” Titiporn said. “It’s not the real mothers.”
Chalida Ekvitthayavechnukul contributed reporting.